NTA UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September: To score better marks in the NTA UGC NET Environmental Sciences, you should have depth knowledge of the entire subject.
You can boost your preparation by referring to NTA UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September. It will give you information about the important chapters and concepts covered in all chapters.
You must have NTA UGC NET Environmental Sciences Solved Question Papers along with the latest Environmental Sciences Syllabus to enhance your semester exam preparation.
Here we have provided you with the complete guide on NTA UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September.
NTA UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September
NTA UGC NET Environmental Sciences Paper 3 Solved Question Papers are the best study materials to score good marks in the Environmental Sciences exam.
Practicing these NTA UGC NET Environmental Sciences paper 3 Previous Year Question papers assist the students in getting a clear idea about the question paper pattern and types of questions asked in the exam.
You can check the complete NTA UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September.
1. In which part of the atmosphere the momentum, heat flux and moisture content are conserved?
(A) Surface Boundary Layer
(B) Mesosphere
(C) Troposphere
(D) Stratosphere
Answer: (A)
2. The soil type which is good for agriculture is
(A) Podozols
(B) Latosols
(C) Serpent soil
(D) Solonachak
Answer: (B)
3. A local laboratory analyzed a water sample and determined that it contained a total solid (TS) content of 132 mg/L and a conductivity of 112 μS/cm. The total suspended solid (TSS) content (mg/L) of water will be
(A) ~ 57
(B) ~ 75
(C) ~ 32
(D) ~ 120
Answer: (A)
4. The settling of discreet, non-flocculating particle in a dilute suspension is known as
(A) Class-I sedimentation
(B) Class-II sedimentation
(C) Class-III sedimentation
(D) Compression
Answer: (A)
5. The theoretical oxygen demand for a solution containing 500 mg/L of phenol will be
(A) 298 mg/L
(B) 596 mg/L
(C) 1191 mg/L
(D) 2382 mg/L
Answer: (C)
6. The compound p-dichlorobenze has been found to have KOM = 625. For a soil containing 1.6% organic matter, the distribution coefficient (Kd) will be
(A) 2
(B) 5
(C) 10
(D) 20
Answer: (C)
7. Which one of the following is the single most important reactive intermediate species in atmospheric chemical processes?
(A) OH.
(B) O2.–
(C) ROO.
(D) OH–
Answer: (A)
8. As per Indian Standards (BIS) for drinking water desirable limit for total hardness as CaCO3 is
(A) 100 mg/l
(B) 200 mg/l
(C) 300 mg/l
(D) 400 mg/l
Answer: (C)
9. Flue gas laden with fine particles from a thermal power plant with a volume flow rate of 100 m3/second passes through an electrostatic precipitator (ESP) having 5000 m2 of collector plate area. If the particle collection efficiency of the ESP is 98%, the drift velocity of the flue gas must be
(A) ~ 0.052 m/s
(B) ~ 0.078 m/s
(C) ~ 0.15 m/s
(D) ~ 1.5 m/s
Answer: (B)
10. Molar extinction coefficient of malondialdehyde is 0.155 mM–1 Cm–1. The concentration of malondialdehyde in a solution having an absorbance of 0.31 is
(A) 2 mM
(B) 0.31 mM
(C) 0.155 mM
(D) 1.55 mM
Answer: (A)
11. Removal of top fertile soil by water is called
(A) Leaching
(B) Siltation
(C) Weathering of soil
(D) Soil erosion
Answer: (D)
12. The rate of settling of air-borne particles in the atmosphere varies with their aerodynamic diameter (d)as
(A) α d
(B) α d2
(C) α d3
(D) α d½
Answer: (B)
13. The smokestack plumes exhibit ‘coning’ behaviour when
(A) Stable atmospheric conditions exist
(B) Atmosphere is unstable
(C) The height of the stack is below the inversion layer
(D) Inversion exists right from the ground surface above
Answer: (A)
14. Among total dissolved matter in marine water, chlorine accounts for
(A) 30%
(B) 55%
(C) 12%
(D) 6%
Answer: (B)
15. Photodissociation of NO2 occurs in the presence of photons of wavelength,
(A) < 0.39 μm
(B) 0.5 – 0.6 μm
(C) 0.6 – 0.65 μm
(D) 0.65 – 0.7 μm
Answer: (A)
16. Peroxyacetyl Nitrate (PAN) is formed by oxidation of
(i) Hydrocarbons
(ii) Isoprene
(iii) Terpene
(iv) Arsenic
Choose the correct answer from the codes:
Codes:
(A) (i) and (iv)
(B) (ii) and (iv)
(C) (iii) and (iv)
(D) (i), (ii) and (iii)
Answer: (D)
17. The evolution of genetic resistance to antibiotics among disease-carrying bacteria is an example of
(A) Directional natural selection
(B) Stabilizing natural selection
(C) Diversifying natural selection
(D) Convergent natural selection
Answer: (A)
18. Pulmonary oedema is caused by
(A) Carbon monoxide
(B) Sulphur dioxide
(C) Nitrous oxide
(D) Methane
Answer: (C)
19. What is the half-life of 131I?
(A) 60 days
(B) 8 days
(C) 12 years
(D) 30 days
Answer: (B)
20. Which one of the following makes blood toxic, after combining with haemoglobin?
(A) CO2
(B) CO
(C) SO2
(D) CH4
Answer: (B)
21. Respiratory electron transport chain can be inhibited by
(A) ADP
(B) Phosphate
(C) H2S
(D) CO2
Answer: (C)
22. Assertion (A): Oil slick in the ocean causes mass scale death of fish.
Reason (R): The gills of fish get clogged.
Point out the correct one of the following:
(A) Both (A) and (R) are true with (R) being the correct explanation.
(B) Both (A) and (R) are true but (R) is not the correct explanation.
(C) (A) is true, but (R) is wrong.
(D) Both (A) and (R) are wrong.
Answer: (A)
23. Chaparral, Maquis, Encinous, Melleseab are important areas of
(A) Tropical evergreen woodland
(B) Temperate evergreen woodland
(C) Tropical deciduous woodland
(D) Temperate deciduous woodland
Answer: (A)
24. The Keystone predator species maintain diversity in a community by
(A) Preying on community’s dominant species
(B) Allowing immigration of other predators
(C) Competitively excluding other predators
(D) Coevolving with their prey
Answer: (A)
25. Which of the following is not an external factor controlling an ecosystem?
(A) Climate
(B) Topography
(C) Parent material forming soil
(D) Microbes
Answer: (D)
26. Which of the following food chain is correct?
(A) Phytoplankton → Zooplankton → Turtle → Crabs
(B) Phytoplankton → Zooplankton → Crab → Turtle
(C) Turtle → Crab → Zooplankton → Phytoplankton
(D) Zooplankton → Turtle → Crab → Phytoplankton
Answer: (B)
27. Which of the following is not categorized as an internal factor of an ecosystem?
(A) Decomposition
(B) Succession
(C) Root competition
(D) Bedrock
Answer: (D)
28. Two species cannot remain in same place according to
(A) Allen’s law
(B) Gause hypothesis
(C) Doll’s rule
(D) Weismann’s theory
Answer: (B)
29. Identify the correct pair:
(A) Ecotope – Transition between two ecosystems.
(B) Edaphic – Saline soil
(C) Heliophytes – Photophilic plants
(D) Ecotone – Particular type of soil
Answer: (A)
30. Based on the number arrange the following group of endemic vertebrate species of India in descending order:
(i) Mammals
(ii) Birds
(iii) Reptiles
(iv) Amphibians
Choose the correct answer from the following:
(A) Amphibians, Reptiles, Birds and Mammals.
(B) Reptiles, Amphibians, Birds and Mammals.
(C) Mammals, Birds, Amphibians and Reptiles.
(D) Birds, Mammals, Reptiles and Amphibians
Answer: (B)
31. Freshwater ecosystems are nutritionally limited by
(A) Phosphorous and Iron
(B) Phosphorous and Carbon
(C) Iron and Nitrogen
(D) Nitrogen and Calcium
Answer: (A)
32. ‘Threshold of Security’ refers to the population level at which
(A) Parasites damage the host body but do not cause immediate mortality.
(B) Predators no longer find it profitable to hunt for the prey species.
(C) Functional response of the predator is very high.
(D) The balance between parasite and host is disturbed as the host produces antibodies.
Answer: (B)
33. “Bermuda grass allergy” is a type of
(A) Airborne allergy
(B) Contact allergy
(C) Hydroborne allergy
(D) Soilborne allergy
Answer: (A)
34. Parasites which initiate production of antibodies within hosts are termed as
(A) Endoparasites
(B) Pathogenic parasites
(C) Zooparasites
(D) Homoparasites
Answer: (B)
35. Which of the following material is not easily broken down?
(A) Cellulose
(B) Hemicellulose
(C) Chitin
(D) Amino acids
Answer: (C)
36. Melting of polar ice is expected to cover a sea level rise of approximately
(A) 10 metre
(B) 20 metre
(C) 60 metre
(D) 100 metre
Answer: (C)
37. Pleistocene represents period of
(A) Cold climate
(B) Warm climate
(C) Alteration of cold and warm climate with high proportion of cold period
(D) Alteration of cold and warm climate with very high proportion of warm period
Answer: (C)
38. GIS is applied to study
(A) View shed analysis
(B) Environmental Impact Assessment
(C) Wildlife habitat analysis and migration routes planning
(D) All of the above
Answer: (D)
39. Tectonic control in landscape evolution is manifested by
(A) Tilted river terraces
(B) Alluvial forms
(C) Increased boulder proportions in the river belt
(D) River meandering
Answer: (A)
40. Difference between mineral resource and reserve is
(A) Reserve implies high degree of economic viability
(B) Resource implies high degree of geological knowledge
(C) Reserve implies high degree of economic viability and high degree of geological knowledge
(D) Resource implies high degree of economic viability and high degree of geological
knowledge
Answer: (C)
41. Characteristic difference between two polar Ice Caps is
(A) Arctic Ice Cap is on land
(B) Antarctic Ice Cap is on land
(C) Both are on land but Antarctic Ice Cap is thicker
(D) Both are on sea but Arctic Ice Cap is thicker
Answer: (B)
42. Laterite represents
(A) Regolith soil
(B) Glacial soil
(C) Black cotton soil
(D) Red soil
Answer: (A)
43. In a whole-rock chemical analysis the dividing criterion between major and trace element on weight percent basis is
(A) 1 %
(B) 0.1 %
(C) 0.01 %
(D) 0.001 %
Answer: (B)
44. The mineral, most resistant to chemical weathering is
(A) Olivine
(B) Quartz
(C) K-feldspar
(D) Biotite
Answer: (B)
45. Assertion (A): Phosphorus cycle is not an exogenic elemental cycle.
Reason (R): Phosphorus cycle does not have a gaseous component.
Choose correct answer:
(A) Both (A) and (R) are true and (R) is the correct explanation of (A).
(B) Both (A) and (R) are true, but (R) is not correct explanation of (A).
(C) (A) is true, but (R) is false.
(D) (A) is false, but (R) is true.
Answer: (A)
46. The El Nino disappears in March and re-appears in
(A) May
(B) August
(C) October
(D) December
Answer: (D)
47. The elemental composition of earth’s crust in the descending order of weight percent is
(A) Silicon >Aluminium> Iron >Calcium
(B) Aluminium> Iron > Calcium >Silicon
(C) Iron > Calcium > Silicon >Aluminium
(D) Calcium > Silicon >Aluminium> Iron
Answer: (A)
48. Bio-oil can be obtained from lignocellulose by
(A) Combustion
(B) Fast pyrolysis
(C) Gasification
(D) Transesterification
Answer: (B)
49. For an ideal Magneto-hydrodynamic power generator, the power output (P) varies with the hot fuel velocity u as
(A) P ∝ u
(B) P ∝ u2
(C) p ∝ u3/2
(D) p ∝ u3
Answer: (B)
50. Assuming that due to large scale change in land use pattern of the world, the earth’s albedo changes from 0.32 to 0.3. If the climate sensitivity factor is 0.5 °C w–1 m2, the change in surface temperature of earth will be (take solar constant S = 1400 w/m2)
(A) 3.5 °C
(B) 0.25 °C
(C) 7.0 °C
(D) 1.5 °C
Answer: (A)
51. Global Warming Potential (GWP) of a greenhouse gas (GHG) is a factor comparing the global warming impacts of
(A) 1 m3 of GHG with 1 m3 of CO2
(B) 1 kg of GHG with 1 kg of CO2
(C) 1 gram mole of GHG with 1 gram mole of CO2
(D) 1 kg of GHG with 1 mole of CO2
Answer: (B)
52. The energy released during combustion of methane is ~ 900 kJ/mol. The carbon intensity of methane is
(A) ~ 0.05 gram C/kJ
(B) ~ 0.013 gram C/kJ
(C) ~ 0.018 gram C/kJ
(D) ~ 1.08 gram C/kJ
Answer: (B)
53. The term B10 implies
(A) Blending of 10 percent biodiesel with 90 percent conventional diesel.
(B) Blending of 90 percent biodiesel with 10 percent conventional diesel.
(C) Blending of 50 percent biodiesel with 50 percent conventional diesel.
(D) Blending of 1 percent biodiesel with 10 percent conventional diesel.
Answer: (A)
54. The validity period of Environmental Clerance after Environmental Impact Assessment is least for
(A) Mining projects
(B) River valley projects
(C) Harbour projects
(D) Area development projects
Answer: (D)
55. In Environmental assessment study, interpretation and evaluation should consider
(A) Uncertainty of possible impacts
(B) Significance of measured impacts
(C) Comparison of alternatives
(D) All of the above
Answer: (D)
56. Who are responsible for the public consultation process of EIA?
(A) State Pollution Control Board
(B) State Pollution Control Board and District Collector
(C) State Pollution Control Board and CPCB Chairman
(D) State Pollution Control Board and Civil Society
Answer: (B)
57. Arrange the following components of an environmental management system in a sequential order. Choose the correct answer from the codes given below:
I. Planning
II. Environmental policy
III. Implementation
IV. Monitoring
V. Review
Codes:
(A) I, II, III, V, IV
(B) II, I, III, IV, V
(C) I, III, II, IV, V
(D) I, V, III, II, IV
Answer: (B)
58. Match List – I with List – II and choose the correct answer from the codes given below:
List – I List – II
a. Life Cycle Assessment 1. 14010 series
b. Environmental Auditing 2. 14030 series
c. Environmental Performance Evaluation 3. 14040 series
d. Environmental Labelling 4. 14020 series
Codes:
a b c d
(A) 1 4 3 2
(B) 3 1 2 4
(C) 2 3 4 1
(D) 4 2 1 3
Answer: (B)
59. Which statement is not correct for hazardous wastes?
(A) They contain one or more of 39 toxic compounds
(B) They catch fire easily
(C) They are nonreactive and stable
(D) They are capable of corroding metal containers
Answer: (C)
60. Right to clean environment is guaranteed in Indian Constitution by
(A) Article 14
(B) Article 19
(C) Article 21
(D) Article 25
Answer: (C)
61. National Ambient Air Quality Standards for major pollutants were notified by CPCB in
(A) 1994
(B) 1984
(C) 2004
(D) 1974
Answer: (A)
62. Public Liability Insurance Act came into existence in the year
(A) 1986
(B) 1989
(C) 1991
(D) 1995
Answer: (C)
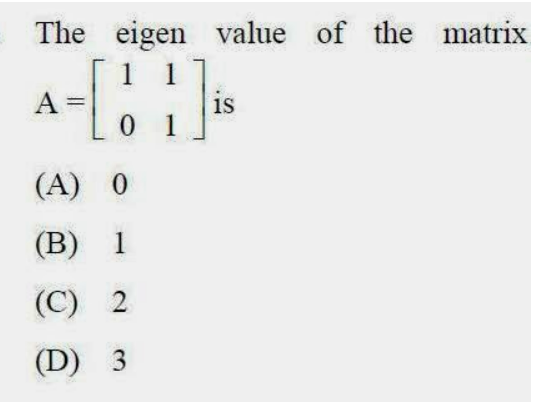
63.
Answer: (B)
64. The standard deviation of weights of certain 1 kg packets of milk is 10 grams. A random sample of 20 packets showed a standard deviation of 15 grams. The value of χ2 statistic for the sample is
(A) 30
(B) 45
(C) 1.5
(D) 0.66
Answer: (C)
65. Assertion (A): According to Gaussian Plume Model, the downward concentration of pollutant appears to beinversely proportional toaverage wind speed at effectivestack height.
Reason (R): Plume rise does not depend on wind speed. It only depends on buoyancy flux parameter.
Identify the correct answer:
(A) Both (A) and (R) are true and (R) is the correct explanation of (A).
(B) Both (A) and (R) are correct and (R) is not the correct explanation of (A).
(C) (A) is true, but (R) is false.
(D) (A) is false, but (R) is true.
Answer: (C)
66. The population of an urban area increased from 5 million to 15 million over a period of 50 years. If the growth of population has been exponential at a constant rate over this period, the growth rate is
(A) ~ 0.693 %
(B) ~ 1.2 %
(C) ~ 1.38 %
(D) ~ 2.2 %
Answer: (D)
67. A random sample of size 26 has a mean of 20. The sum of squares of the deviations taken from the mean is 200. If the population mean is 18, what is the value of t-statistic?
(A) ~ 0.9
(B) ~ 2.1
(C) ~ 3.6
(D) ~ 5.2
Answer: (C)
68. In Y-shaped energy flow model, one arm represents herbivore and the other
(A) Carnivore
(B) Decomposer
(C) Omnivore
(D) Secondary consumer
Answer: (B)
69. The population of a certain fish species in a pond follows logistic equation dN/dt = αN – βN2. When α = 0.5 and β = 0.01, the maximum sustainable yield is
(A) 25
(B) 50
(C) 20
(D) 5
Answer: (A)
70. A change in a population’s gene pool over time is called
(A) Microevolution
(B) Macroevolution
(C) Chemical evolution
(D) Inorganic evolution
Answer: (A)
71. The scale length of variation of pressure in vertical direction in atmosphere is
(A) ~ 2.5 km
(B) ~ 5 km
(C) ~ 7 km
(D) ~ 8.5 km
Answer: (C)
72. Assertion (A): Planetary, synoptic and mesoscale motions in earth’s atmosphere are essentially quasi-horizontal.
Reason (R): The vertical component of wind velocity is more than an order of magnitude smaller than its horizontal component for all motions in atmosphere.
Identify the correct answer:
(A) Both (A) and (R) are true and (R) is the correct explanation of (A).
(B) Both (A) and (R) are true and (R) is not the correct explanation of (A).
(C) (A) is true, but (R) is false.
(D) (A) is false, but (R) is true.
Answer: (A)
73. GLOBE stands for
(A) Global Leading Occupations to Benefit the Environment
(B) General Learning and Observations to Benefit the Environment
(C) Global Learning and Observations to Benefit the Environment
(D) Global Leaders and their Observations to Benefit the Environment
Answer: (C)
74. Which one of the following is not the goal of sustainable agriculture in India?
(A) Maintaining productive topsoil
(B) Reduce the use of chemical fertilizer and pesticides
(C) Mechanised farming
(D) Keep farms economically viable
Answer: (C)
75. The term of Kyoto Protocol has been extended beyond December 2012 by
(A) 5 years
(B) 7 years
(C) 8 years
(D) 3 years
Answer: (A)
Year Wise Solved UGC NET Environmental Sciences Paper 3 Previous Year Question Papers
The old UGC NET Examination paper-3 in Environmental Sciences was of descriptive type till December 2011.
paper-3 becomes objective type from June 2012 to November 2017. From July 2018 onward, paper-3 was stopped and becomes part of paper-2 itself.
So, the old questions for paper-3 from June 2012 to November 2017 which were of objective type (multiple choice questions) can be used by the UGC NET aspirants for their better preparation for paper 2.
Year Wise NTA UGC NET Environmental Sciences Paper 3 Solved Question Papers are given below.
| Download Year Wise NTA UGC NET Environmental Sciences paper 3 Solved Paper |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2017 November |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2016 July |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2015 June |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2015 December |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2014 June |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2014 December |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 December |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 June |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2012 December |
| UGC NET Environmental Sciences Paper 3 Solved Question Paper 2012 June |
We have covered the NTA UGC NET Environmental Sciences Paper 3 Solved Question Paper 2013 September.
If you have any questions about NTA UGC NET Environmental Sciences Paper 3 Solved Question Papers, please let us know in the comment section.